Improving Profits and Reducing Compliance Costs
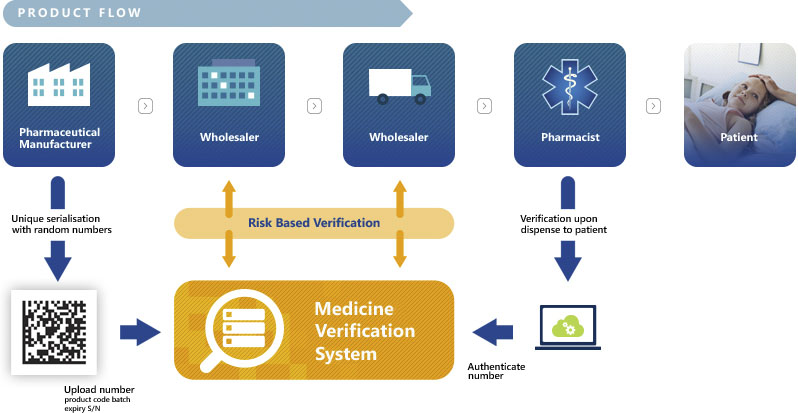
Track and Trace, Serialization Made Easier
Today's pharmaceutical and nutriceutical companies have to meet exacting requirements from suppliers, customers and government regulators and Technologies International, inc. helps businesses meet those standards.
We've developed solutions that directly integrate both Track-and-Trace and serialization directly into SYSPRO ERP and WMS systems,
Our solutions provide robust serial warehouse capabilities and cloud solutions, enabling error proof customer supply chains and real-time tracking of meaningful business metrics.
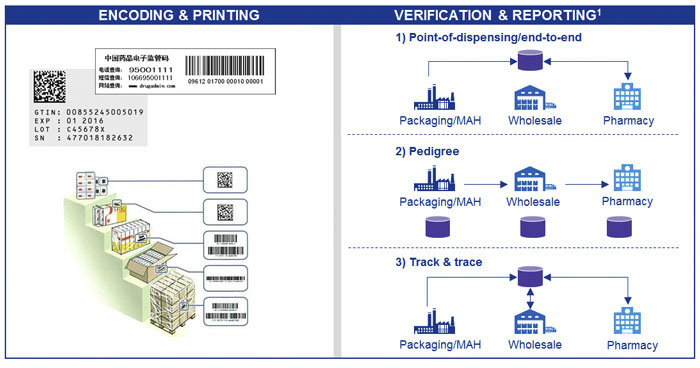
Track and Trace
At Technologies International, inc. We understand that track and trace solutions deliver far more than just economic benefits and supply-chain efficiencies. the benefits extend far beyond that.
Experts estimate that 5% of all drugs sold worldwide are counterfeits – in some countries, that figure can go as high as a shocking 50 %.
Studies have shown that billions of dollars in products are “lost in the supply chain” every year.
Our customers recognize that counterfeit drugs and product diversion can risk lives, cut deeply into their revenues, and threaten their company's reputation.
That's why we work with forward-thinking companies in the pharmaceutical and nutriceutical industries to ensure supply-chain security, give them the ability to authenticate ePedigrees-the life history of products.
The Technologies International, inc. track and trace solutions are an essential part of this strategy. We've integrated track and trace solutions directly into the SYSPRO ERP system so that our customers can quickly and efficiently identify the origins of a pharmaceutical industry product and verify its authenticity.
With the Technologies International, inc track and trace solution in place client can fight back against product diversion that impacts everything from licensing obligations to distribution agreements and revenues.
Technologies International, inc. works directly with pharmaceutical and nutriceutical clients to deliver customized solutions to help companies stay on top of market trends as well as meet all legal and regulatory requirements.
Our integrated and comprehensive track and trace solutions, cover all stages of the global life science industries supply chain and it's designed to optimize all operational areas while securing and improving your competitiveness on a long-term basis.
Serialization
The global pharmaceutical industry is moving towards a serialised world.
Increasing threats to patient safety from counterfeited and diverted pharmaceuticals are driving more stringent regulations and pharmceutical and nutriceutical companies have to stay ahead of the changes . Non-compliance is not an option.
Technologies International, Inc. has partnered with industry experts to build serialization strategies directly into ERP and WMS systems, allowing full compliance and cost reductions from beginning to end.
Serialization begins before raw ingredients even arrive. With Technologies International, you can be assured that everything from raw ingredients to the smallest pill shipped to customers is labeled and serialized to provide scrupulous tracking at all stages of the product life-cycle.
Encoding and Printing Serialized Labels
With Technologies International, serialized labeling begins the moment a job is created and it's integrated directly into the ERP system.
Separate labeling, prone to errors and misinformation are eliminated, reducing costs and increasing ROI.
All labels are fully compliant to ISO and GS1 standards.
Verification and Compliance Reporting
No system would be complete without full verification and reporting and Technologies International, Inc and its partners have automated the process to make compliance easier and faster than ever before.
New regulations now require companies to report a mix of master data, packaging and serialisation information or supply chain transaction events to a centralized database where it will be authenticated against the same repository as the labelled medicine that arrives at the pharmacy.
Meeting the Challenges of Track and Trace and Serialization Programs
There are five core challenges that we help pharmaceutical and nutriceutical companies address to implement track-and-trace and serialization program.
- Understanding the regulatory environment and requirements
Track-and-Trace and Serialization programs typically start with understanding regulatory requirements, impacts and goals.
Before we begin we work with with companies to outline regulatory challenges, business goals, timelines and the scope of work and then we build business models to facilitate those outcomes. - Managing Cross-functional Teams Change doesn't happen in a vacuum and track-nd-trace and serialization's impacts aren't limited to labels, line equipment and line systems.
For a successful implementation, we work with our clients to build cross-functional teams to train and build support based on the current capabilities of people, processes, technology and business partners.
- Avoiding Redundant Processes And Work
A new system and requirements mean a re-evaluation of all processes as well as detailed roadmaps to make it happen.
The Technologies International team collaborates with our customers to make sure that priorities are set, processes created and a detailed road map is created to ensure that best practices become core to any changeovers. - Build a Scalable Track-and-Trace and Serialisation data platform
The track-and-trace and serialization strategies needs to be translated into a solution architecture and pilot programs created to serve as a blueprint for gathering both data (serial numbers) and events about the items coming off the packaging line.
Before any programs go on-line everything needs to be tested and re-tested to make sure the system delivers the desired outcomes. - Leveraging Business Case Studies
Track-and-trace and serialization programs monitoring doesn't end when the new system and processes come on-line. The system has to be checked and re-checked to make sure that it not only complies with all regulations but that it's also delivering the expected outcomes for all areas of the business.

